#25 Diuresis & Negative Fluid Balance
Diuretics are essential tools used by clinicians to manage volume status in ambulatory patients and in the critically ill… but are we using them correctly? Join Nick & Cyrus as they explore principles of fluid balance and teach a masterclass on how to approach diuresis in the critically ill patient. You won’t want to miss this high-yield episode discussing an evergreen topic in the world of critical care medicine!
Quick Take Home Points:
Edema is common in critical illness, but it isn’t benign. Edema results in longer duration or mechanical ventilation, impaired medication absorption, limited mobility. Every liter of cumulative fluid balance is associated with increased mortality.
Avoid fluid overload but following the 5 Strategies to Prevent Fluid Overload:
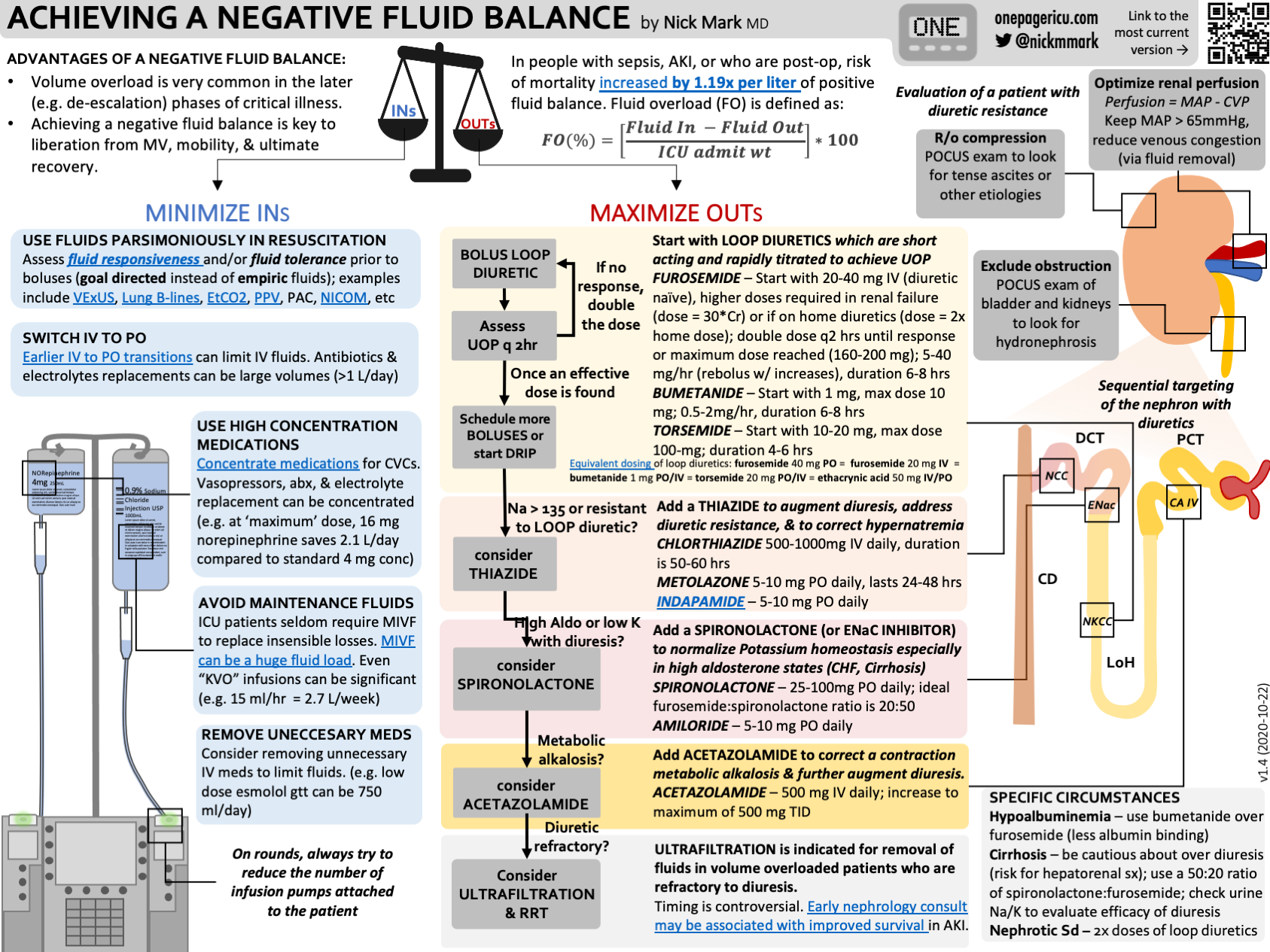
Bolus parsimoniously (e.g. determine fluid responsiveness)
Make no maintenance IV fluid your default, except in rare cases
Always remove unnecessary medications
Whenever possible, switch medications from IV to enteral route
Concentrate all required IV medications
Optimize Renal Perfusion Pressure (RPP = MAP - CVP) by maintaining MAP and reducing CVP with diuresis. Consider a higher MAP goal in selected patients.
Use LOOP diuretics as first line agents. Be sure to use a high enough dose to reach the threshold. In some circumstances loop diuretic infusions can be more effective but remember to bolus when changing dose! Use bumetanide instead of furosemide in people with low albumin. Consider albumin boosted diuresis in select patients with low albumin (always use concentrated albumin).
Add a THIAZIDE in people with low urine sodium who are not meeting diuresis goals.
Add SPIRONOLACTONE to address hypokalemia and to boost diuresis
Add ACETAZOLAMIDE to mitigate contraction alkalosis.
Remember that diuretics only cause renal injury with overdiuresis (e.g. pre-renal AKI).
Infographic:
ICU OnePager Infographic on How to Acheive a Negative Fluid Balance
Show Notes:
Why edema & a positive fluid balance are bad
Edema is very common in people with critical illness. (In one study 40% of ICU patients had subcutaneous edema) Despite it’s frequency edema not benign:
Edema causes many problems:
Edema of the head neck —> Vocal cord, airway edema → loss of cuff leak → potential for extubation failure or failure to extubate
Lungs, pleura → pulmonary edema, pleural effusions → hypoxemia
Edema of the GI system:
Bowel wall edema → impaired medication absorption
Ascites → hypoventilation, impaired absorption
Renal Edema → worsening renal dysfunction
Soft tissue edema → pain, limited mobility, risk for skin infection, also increased likelihood that someone will be discharged to a nursing facility due to difficulties performing IDLs and IADLs.
A positive fluid balance is associated with worse outcomes, including longer duration of mechanical ventilation, longer ICU stay, and greater mortality.
Even though more fluid resuscitation is associated with higher severity of illness, several analyses suggest that positive fluid balance is an independent predictor for poor outcomes.
In one large meta-analysis of 31 observational studies, the cumulative fluid balance after 3 days was strongly predictive of ICU mortality.
The risk of mortality increased by a factor of 1.19 (95% CI, 1.11-1.28) per liter increase in positive fluid balance.
This was particularly true in patients with sepsis, respiratory failure, acute kidney injury.
In one large study, (SOAP) the magnitude of positive fluid balance was the single best predictor of ICU mortality.
Many patients require significant fluid resuscitation early in critical illness, but it is important to be purposeful with the resuscitation, not reflexive. Use of techniques to determine fluid responsiveness is important. Equally important is deliberately transitioning from a resuscitation to de-resuscitation once the patient has stabilized.
“If you were woken up because someone’s pressures were low, you wouldn’t reflexively give another gram of vancomycin and go back to sleep. You probably shouldn’t reflexively give fluid boluses either.”
5 Strategies to PREVENT volume overload
1. BOLUS IV Fluids PARSIMONIOUSLY in resuscitation
Instead of just giving fluids and seeing what happens, we should be using a method to determine if the person is fluid responsive and tolerant.
Be deliberate not reflexive. Don’t repeat boluses if ineffective. If someone did not respond to a fluid bolus, it is unlikely to be beneficial if repeated.
Carefully consider the choice of fluid. Would blood products or albumin be appropriate?
Consider the phase of illness, if someone is a week into their hospital stay and already several liters positive, they are much less likely to require additional volume.
2. Make NO MAINTENANCE IV FLUIDS Your DEFAULT
Most patients do not require maintenance IV fluids (MIVF).
If someone is drinking they do not need MIVF
If someone is intubated, consider starting tube feeds instead of IV fluids
If someone is NPO for a procedure, they generally do not require MIVF during the brief interval before.
Also remember that according to the ASA guidelines for healthy, non-pregnant patients, they only need to fast for 2 hours after clear liquids.
There are some situations the do require more aggresive fluid resuscitaiton. For example, DKA, burns, severe diarrhea, etc. But these are the minority of ICU patients and even these patients seldom require MIVF for more than 24-48 hours.
Much Better to err on the no MIVF side and add them later, rather than to give too much upfront and need to get rid of them later.
3. Always REMOVE Unnecessary Medications
Stopping unnecessary meds is a great habit to get into. In particular, being fastidious about antibiotics, often means stopping vanc 12 or 24 hours sooner once you see that MRSA NAAT is negative. This can save liters of fluid.
The best way to actualize this is to do “ID rounds” in the afternoon/evening, where you see if any new results have come up and if you can narrow/remove antibiotics.
Keep vein open (KVO) fluids are also often unnecessary, particularly if running into a central line. In many cases, if a line is not being used for continuous infusion of a medication it can be either heparin locked or flushed every few hours. This can also reduce volumes administered.
4. SWITCH Medications from IV to enteral whenever possible
For medications that are necessary, always ask yourself can this be given enterally?
Intravenous electrolyte replacement can easily result in hundreds of mL of additional fluid each day. Whenever possible convert these to enteral.
In addition to avoiding hundreds of mLs of volume, remember that enteral medications are much cheaper, less work for the RN to administer, and come with zero risk of bloodstream infection.
5. CONCENTRATE ALL IV MEDS that remain
For people who truly are critically ill, who need to be on intravenous meds and who can’t absorb those meds enterally many will have central access. This means you can concentrate meds for central lines.
You should ALWAYS concentrate vasopressors, antibiotics, and electrolytes.
For example:
If someone is on “maximum” dose of 30 mcg/min of norepinephrine using the “standard” concentration of 4 mcg/ml they are getting a lot of fluid. Well over 100 ml per hour. (>2 liters per day)
If you switch them to “quad strength” norepinephrine (16 mcg/mL) you can save over 2 liters of fluid per day!
Bonus: for many meds you can concentrate meds in either NS or D5W. This is an opportunity to adjust for hypo or hypernatremia.
“RPP = MAP - CVP”
The key concept of renal perfusion pressure
Renal perfusion pressure (RPP) = MAP - CVP
Consider two examples:
Normal healthy individual:
BP 120/80 means a MAP of 93, CVP might be 3, so kidneys are receiving ~90 mmHg of perfusion pressure. More than enough for them to work properly
Now let’s think about someone with volume overload in the ICU:
MAP of 65 with a CVP of 20, means the RPP is only 45 mmHg
This person is getting HALF the renal perfusion of the healthy person due to venous congestion.
From a hemodynamic perspective, this is the same as having a MAP of 45 with a CVP of 0 mmHg (which most people would recognize as a true emergency!)
Take away points:
When optimizing renal function, always consider the renal perfusion pressure.
Recognize that venous congestion and arterial hypotension can both compromise renal perfusion. In some cases, diuresis is the best strategy to improve renal perfusion because it improves venous congestion.
Debunking the myth that “CVP isn’t useful”
Many people have heard that CVP isn’t useful. This is partially true. CVP isn’t useful to predict fluid responsiveness. (There are much better tools available to determine fluid responsiveness)
CVP was formerly used to guide crystalloid resuscitation in the early goal directed therapy (EGDT) era. This resulted in far too much fluid being given. See the discussion on parsimonious fluid resuscitation for more.
See this excellent article for a thorough discussion.
But CVP is very useful to understand RPP (and CPP). CVP is also somewhat useful to understand RV preload.
If you can exclude confounders (RV dysfunction, TR) using POCUS, you can interpret a high CVP as being more representative of filling pressures.
If you can compare CVP to a patients baseline it can provide additional information if it changes. (e.g. a sudden rise in CVP can suggest tamponade or RV failure)
In summary, don’t draw vast conclusions from half vast data. CVP is just one datapoint. CVP is useless for assessing fluid responsiveness, however it can provide very useful information about venous congestion, and with appropriate context it can be useful to evaluate RV filling pressures and RV function. Use it wisely!
— Nick Mark MD (@nickmmark) February 17, 2024
Approach to diuretic therapy in the ICU
Diuretics Options:
LOOP DIURETICS (FUROSEMIDE, BUMETANIDE) work in the NKCC channels in the distal Loop of Henle. This causes sodium and potassium to be lost, and with them water. These are the primary & first line diuretics used in the ICU.
The biggest side effect is hypokalemia
Also need to be aware of ototoxicity when given too fast.
THIAZIDE DIURETICS (CHLORTHALIDONE, METOLAZONE, HCTZ) works on NCC channels in the distal convoluted tubule. This is important because, often with sustained loop diuresis there is a compensatory increase in distal reabsorption, which can make loop diuretics less effective. Thus thiazides are particularly useful to address diuretic resistance.
ALDOSTERONE ANTAGONIST (e.g SPIRONOLACTONE) works on ENaC channels, also in the DCT. Like thiazides, aldosterone antagonists can be effective if there is compensatory distal reabsorption making your loop less effective. It’s also a so called “potassium sparing diuretic” because they cause natiuresis without kaliuresis… thus they are useful in combination with loop diuretics to balance out hypokalemia.
CARBONIC ANHYDRIDE INHIBITOR (e.g. ACETAZOLAMIDE) work on carbonic anhydrase in the proximal convoluted tubule (they also inhibit CA in other parts of the body, which can cause side effects). Inhibiting CA enhances secretion of bicarbonate and also to a lesser extent water. This is particularly useful to address contraction alkalosis.
An algorithm for diuresis
Start with a LOOP DIURETIC
In someone who is diuretic naive, furosemide 40 mg IV is a reasonable starting point
In people who take loop diuretics, consider doubling the dose.
Bumetanide is valuable in people where furosemide isn’t working, particularly in people with low albumin.
In order to get to the nephron, furosemide is bound to albumin. Bumetanide is bound to other serum proteins. Therefore in people with low albumin (e.g. nephrotic syndrome) it is reasonable to try bumex instead of lasix.
There is good evidence that bowel wall edema reduces the efficacy of oral loop diuretics. One study actually measured bowel wall thickness using ultrasound and found that greater colon wall thickness predicted oral diuretic failure.
Loop diuretics exhibit a threshold and ceiling dose.
The crucial concept with loop diuretics is that they have almost no effect until you reach a threshold dose. For a healthy person the threshold dose might be just 20 mg IV lasix. For someone with CKD a much higher dose may be required, potentially 100 mg of lasix or more.
A common mistake that people make is that they give a dose below threshold and decided that the person is not-responding to loop diuretics.
There’s also a ceiling dose, the point where you’ve saturated all the NKCC channels and adding more diuretic doesn’t achieve any more urine sodium excretion.
Sometimes people make the mistake of taking someone who is diuresing and trying to increase the dose further. This may not work if you have already hit the ceiling dose.
IV bolus versus continuous infusion of loop diuretics
Continuous infusion is not clearly superior. According to a meta-analysis of 9 studies compared to bolus diuretics, continuous infusion was associated with no difference in UOP, LOS, or mortality.
Also no difference in rates of hypokalemia.
There are advantages to a continuous infusion compared with a bolus:
Inherently goal directed (e.g. how many mL UOP per hour)
RN controlled instead of MD controlled → more likely to hit those goals
We tend to reach higher doses with infusions than with boluses, so we may be more likely to reach the threshold dose. Recall that 20 mg/hr of lasix is the equivalent of 120 mg boluses every 6 hours.
Disadvantages of continuous infusions of loop diuretics:
Uses up a PIV or CVC lumen for infusion
Attaches someone to IV pump → may limit mobility
Very important when using a continuous infusion: you NEED to BOLUS when changing dose.
Recall from pharmacology that you reach steady state of a drug after 5 half lives. When we are giving continuous infusions we are trying to get to steady state to maintain a drug level about the threshold dose.
Furosemide (Lasix) has a 6 hour half life - in fact that’s why it’s called Lasix: it lasts six!
That means that if you go from 10 mg an hour to 20 mg an hour, that change will take effect 30 hours later.
Instead it makes sense to give a bolus in addition to the rate change, that way you reach the new steady state immediately.
Bottom line: drips are not necessarily stronger, but they do have some advantages like more rapid dose escalation, more likely to notice sooner if we are not hitting our goals. But if you are gonna use a drip be sure to bolus when you start it and when you increase the dose!
Be sure to monitor potassium during aggressive diuresis
Minimum twice a day checks, in some cases up to q6Hrs
Adding a Second/Third diuretic
Choice of a second diuretic often depends on the context:
If the sodium is minimal urine output on high dose loop diuretics - e.g. diuretic resistance - or the sodium high (>135) consider adding a THIAZIDE next (metolazone or chorthiazide).
It’s also reasonable to check a urine sodium. If the urine sodium is very low, it suggests distal reabsorption of sodium. This suggests that a person will respond well to a a thiazide
If the potassium is very low or there is a high aldosterone state, consider adding an EnAC inhibitor (SPIRONOLACTONE)
If there is brisk diuresis with metabolic alkalosis - a so called contraction alklalosis - consider adding a carbonic anhydrase inhibitor (ACETAZOLAMIDE)
Special circumstances
Cirrhosis: Because the liver breaks down aldosterone, chronic liver dysfunction aka cirrhosis is a high aldosterone state. That’s why people with cirrhosis tend to respond very well to a combination of loop diuretics and spironolactone.
This also has the advantage of being “eukalemic” if you use the right ratio: Typically 50 mg of PO spironolactone for 20 mg of PO furosemide
Low albumin: because furosemide must be bound to albumin to be transported to the nephron, in people within low albumin it may be less effective. In these people I typically use bumetanide instead.
Also remember that in the case of nephrotic syndrome, you may need very high doses of bumex.
“Albumin Boosted Diuresis”:
The theory is that giving albumin “pulls fluid into the vasculature” which can help with diuresis. The idea is that a small bolus of albumin results in a net increase in plasma volume which results in a net diuresis (e.g. urine output increased by more than the volume of albumin).
Mechanistically (and based on this limited literature) it’s reasonable to consider giving albumin in combination with loop diuretics in critically ill patients who need more effective diuresis AND have a serum albumin in the 2.0-2.5 g/dL range.
There is a limited literature in support of this practice:
A 2021 meta-analysis demonstrates suggests a modest benefit in nephrotic syndrome, especially in those with an albumin of <2.5 g/dL. Based on 13 studies with around 420 participants they demonstrated an average increase in UOP by about 30cc/Hr.
Other reviews and meta-analyses either hint at a possible benefit in this population or highlight the fact that there is tremendous heterogeneity amongst the present studies and more research needs to be done.
Crucially, you should only use concentrated (20% or 25%) albumin NOT 5% albumin if attempting albumin boosted diuresis.
Based on a study in healthy volunteers, administration of 219 ± 33 ml of 20% albumin expands plasma volume by about 360 ml at 1 hour.
In contrast, administration of 877 ± 132 ml of 5% albumin only expanded plasma volume by about 540 ml at 1 hour.
Thus only concentrated albumin results in net plasma expansion.
Addressing diuretic resistance
1st: Rule out obstruction
Renal US - rule out stone or hydrocephalus
Troubleshoot Foley catheter issues - does it flush? How much volume in bladder?
2nd: Stop potentially offending Medications
Stop NSAIDS (obv), consider others such as probenicid
3rd: Optimize Hemodynamics
In general a MAP of 60 or 65 is adequate but not in everyone.
Recall the The 2014 Sepsis and Mean Arterial Pressure (SEPSISPAM trial), randomized 776 patients with septic shock on vasopressors to either a goal MAP of 65-70 or 80-85 mmHg for up to 5 days. There was no difference between the two groups for all-cause mortality at 28 or 90 days, but for those with a history of chronic hypertension there was less renal dysfunction with a higher MAP goal. The higher MAP goal was generally well tolerated, except for an increase in Atrial Fibrillation.
Therefore it’s reasonable to consider a trial of MAP 80 in select patients (those with pre-existing hypertension) who are in oliguric AKI.
4th: Rememebr the Number 1 reason: you aren’t trying hard enough!
Higher dose, given more frequently (or continuously)! Add a second/third agent.
Audio
Video
-
Teran F, Prats MI, Nelson BP, et al. Focused transesophageal echocardiography during cardiac arrest resuscitation: jacc review topic of the week. J Am Coll Cardiol. 2020;76(6):745-754.
Arntfield R, Lau V, Landry Y, Priestap F, Ball I. Impact of critical care transesophageal echocardiography in medical-surgical icu patients: characteristics and results from 274 consecutive examinations. J Intensive Care Med. 2020;35(9):896-902.
-
-
Undifferentiated Shock
Cardiac Arrest