#37 Acute Respiratory Distress Syndrome
On this week’s episode, Cyrus & Nick tackle one of their biggest challenges yet: Acute Respiratory Distress Syndrome, more commonly known as ARDS.***WARNING*** this is no shorty! We cover all things ARDS from pathophysiology, diagnosis, treatments and things NOT to do. Dare we say, this is the definitive FOAM-ARDS experience for anyone and everyone who cares for people suffering from ARDS. Give it a listen and as always, send us your feedback!!
What is ARDS?
Acute respiratory distress syndrome (ARDS) is an acute diffuse lung inflammation characterized by poor oxygenation and reduced compliance.
ARDS is a common cause of respiratory failure - and mortality - in people with critical illness.
ARDS has many causes:
Direct lung injury: e.g Pneumonia, Aspiration, Near-drowning) and in particular lung infections like pneumonia influenza, COVID, etc
Indirect lung injury: e.g. Septic shock, cardiac arrest, drug overdose, pancreatitis, transfusions, etc
You can think of ARDS as a final common pathway where many different insults can lead to a common pathway of lung inflammation; like how septic shock can be caused by infection with different organisms at different sites.
Pathophysiology of ARDS
An insult leads to inflammation-mediated disruptions in alveolar-capillary permeability.
This causes fluid to spill out into the alveolar space, resulting in edema. The collection of proteinacious fluid in the alveoli leads to alveolar collapse/derecruitment. This causes the characteristic reduction in compliance.
Simultaneously we get impaired gas exchange due to shunting and ventilation-perfusion mismatch.
We also frequently get a rise in pulmonary vascular resistance as blood redirected away from collapsed areas.
For more about understanding hypoxemia, see our earlier episode:
Lung injury in ARDS follows several phase:
Exudative phase - occurs within the first 7 days, and is characterized by damage to the lung's alveolar epithelium and vascular endothelium, which leads to fluid, protein, and red blood cells leaking into the lungs.
Proliferative phase - begins 2–7 days after the initial lung injury. It's characterized by the proliferation of type 2 pneumocytes, the development of early fibrosis, and the thickening of the alveolar capillaries.
Fibrotic phase - This phase is not reached by all patients, but in those who do, it's characterized by the development of scar tissue in the lungs. It's associated with increased collagen deposition, prolonged ventilation-perfusion mismatching, and further diminishment of lung compliance.
ARDS is a heterogeneous disease, and many authors have argued that there are ‘sub-phenotypes’ that benefit from personalized therapies:
Fluid responsive patients with ARDS - arguing for a liberal vs conservative fluid strategy
“Recruitable lung” e.g patients who respond well to PEEP -
Those who have a “hyper-inflammatory” phenotype such as those with COVID-19 as the cause for their ARDS - potentially those who benefit most from anti-inflammatory medications (e.g. corticosteroids).
In a recent publication by Siuba and colleagues they took this concept one step further and demonstrated a high PEEP strategy in those who have less of an inflammatory response and lower severity of illness actually have increased mortality when a high PEEP strategy is implemented.
“Every patient is an n of 1”
Definition of ARDS
The new Global Definition of ARDS
A diagnosis of ARDS requires 4 criteria to be met:
Acute onset: Symptoms must appear within one week after some insult.
Bilateral opacities on imaging: typically chest radiography or CT - must show bilateral opacities. POCUS can also be used.
Edema origin is not primarily cardiac: Edema must not be fully explained by cardiac failure or fluid overload. Cardiac causes can be present, but must be judged to not be a
Hypoxemia is present - usually defined by the P/F ratio. The P/F ratio or PaO2/FiO2 ratio is also how we grade the severity of ARDS in the US, though in resource limited settings SpO2/FiO2 can be used.
It is important to understand that this is broad pragmatic definition, meant to be as inclusive as possible. The definition has been changed several times, each time becoming slightly broader.
Earlier definitions required the patient to be intubated. (The current definition allows HFNC or NIPPV).
Earlier definitions required arterial blood gas to confirm hypoxemia (the current definition allows S/F ratio).
Earlier definitions used PCWP to exclude cardiac causes. (The current definition does not)
Does it matter that the definition of ARDS is broad?
On one hand it really doesn’t - the definition of ARDS is largely a research tool, which is important for studies, but less important for clinicians at the bedside.
But on the other hand, it probably is good to have a broad flexible definition for one reason:
ARDS has high mortality and morbidity
There are several highly effective treatments; but they are applied inconsistently and often too late in someones course.
Simply asking “could this person have ARDS” potentially triggers life-saving interventions, much like how “could this person be septic does”
For that reason having a broader more inclusive definition is probably a good thing.
Disease vs Syndrome, Diffuse Alveolar Damage, and Pseudo-ARDS
What does it mean to be a “syndrome” instead of a “disease”
Many diseases are defined by what cells look like under a microscope or even what mutations are present. But ARDS is defined more loosely, based on clinical criteria.
As a result ARDS is a heterogeneous group of patients - some with severe disease and others with more mild disease, who are all lumped together.
Diffuse alveolar damage (DAD) is the histological hallmark of ARDS. It’s acute phase with edema, hyaline membranes, and inflammation. It’s followed by an organizing phase with type II pneumocyte hyperplasia and fibrosis.
If we did lung biopsies - which we don’t because it would be incredibly morbid - only about half of the patients with ARDS have diffuse alveolar damage on pathology.
Does this mean we are getting the diagnosis wrong half the time? NO
It means that like many conditions in the ICU (AKI, sepsis, etc), ARDS is heterogeneous! There is a spectrum of disease present.
“Performing a lung biopsy in critical illness is like checking your credit score. The very act of checking makes it worse.”
Even though we don’t focus on the pathological findings as much as the clinical findings, we DO often perform bronchoscopy with BAL. This is really important for a few reasons:
If the cause of ARDS is infectious, it helps us identify the microbe and tailor our therapy.
There are several conditions that are ARDS mimics - disease like acute eosinophilic pneumonia which can look like ARDS but require different treatment.
See an upcoming episode for more:
Pseudo-ARDS is loosely defined as ARDS that improves very rapidly with treatment and does not follow the expected course of several weeks.
Is this a misdiagnosis like cardiogenic pulmonary edema? Possibly
Does it matter? Maybe Not. ARDS vs pseudo-ARDS is a bit of a distinction without a meaningful difference. If someone meets criteria for ARDS, treat them accordingly. If they get better fast, great! Whether it was ARDS or something like ARDS doesn’t really matter
The 11 P’s of Treatment
Protective ventilation
PEEP
Prone positioning
Prednisone (really dexamethasone)
Paralytics (really neuromuscular blockers)
Prostacyclins (inhaled EPO and inhaled nitric oxide)
Peripheral oxygenation (vvECMO)
Parsimonious fluids
Peeing
Pleural Evacuation
Palliation
ICU OnePager about ARDS.
1. Protective Ventilation / PEEP
In order to understand lung protective ventilation, we need to understand the mechanisms where the ventilator can cause lung injury; or ventilator induced lung injury (VILI).
Barotrauma - too much pressure
Volutrauma - too much volume
Atelectrauma - injury from atelectatasis or alveoli popping open and closed
Mitigated by the so-called “open lung” strategy whereby PEEP is used to prevent alveoli from repetitive opening and closure
Biotrauma - could be thought of as the produce of the aforementioned traumas leading to an increase in inflammatory mediators that may mechanistically explain the concept of VILI - vent induced lung injury
Hyperoxia - idea that too much oxygen can cause oxidative damage.
LPV seeks to minimize this damage by limiting pressure & volume, while using positive expiratory pressure (PEEP) to keep the lung inflated, and avoid popping alveoli open and closed (atelectrauma). Additionally, FiO2 is gradually titrated and the lowest concentration necessary is selected.
The key trial was ARMA or ARDSNet trial (NEJM 2002)
It compared 6 ml/kg PBW with plateau <30 cmH2O with “standard of care” 10-12 ml/kg with plateau <50 cmH2O
Stopped early for benefit
Mortality: 31.0 percent vs. 39.8 percent, P=0.007
8.8 Absolute reduction in mortality, which is about as good as any ICU study has ever been.
Importantly those “standard of care” settings (VT = 10-12 ml/kg) are insanely large.
2. PEEP and Driving Pressure
Although ARDSNet has a protocolized way to titrate PEEP, it’s possible to use Driving Pressure to make smarter PEEP choices.
Driving pressure (∆P) = Pplat - PEEP
A higher ∆P indicates a lower compliance.
Driving pressure therefore can tell you if you are overdistending or potentially damaging the lung.
High quality observation studies suggest that decreasing driving pressure may improve outcomes for patients requiring mechanical ventilation.
A patient level meta-analysis of 9 RCTs of n=3562 patients, found that driving pressure was the single best predictor of mortality.
Specifically, achieving a Driving pressures (∆P) of < 15 cmH2O has demonstrated the greatest benefit in terms of mortality.
Prospective studies of driving pressure defined ventilation in ARDS are ongoing.
It is reasonable to choose your initial settings using ARDSNet Guidelines, but choose the optimum PEEP setting by comparing two or more settings and seeing which achieves a lower driving pressure.
Driving Pressure & Mechanical Power Calculator
3. Prone Positioning
Prone positioning or “proning,” improves oxygenation, and according to a 2013 NEJM study found that if implemented early, proning resulted in reduced 30 and 90 day mortality. Subsequent meta-analyses of 8 RCTs have confirmed this benefit on survival.
Practically, this means that intubated patients are rotated face down for ~18 hours per day, then back supine for 6 hours or so. This “supination” important to prevent pressure injuries.
This can be done without any special equipment, just careful coordination of a team. Special care is essential to prevent ETT dislodgement. It is prudent to place CVC and arterial line prior to proning.
Physiology of prone positioning:
When we are supine, our heart compresses the posterior aspects of our lungs. By rotating them prone, the heart is pressing against the sternum not the lungs.
Another factor is that in ARDS, dependent areas of the lung become edematous. By spending part of your day on your back and the rest on your front, you distribute this edema, which ameliorates it somewhat, improving gas exchange.
The definitive study of prone positioning was PROSEVA (NEJM 2013)
Myths about prone positioning:
“Proning didn’t improve hypoxemia” - Sometimes we try prone and it doesn’t appear to improve oxygenation, so we conclude that “it didn’t help.”
A sub-group analysis of the PROSEVA study found that even in people whose oxygenation didn’t improve there was still an improvement in survival. This suggests that we probably should be doing proning more often, even if we don’t see an improvement in oxygenation.
“Stopping tube feeds” - Another myth that we should bust about prone positioning, is that you don’t need to stop tube feeds while prone. This is wrong. In fact, the risk of aspiration may even be lower in the prone position.
Awake Prone Positioning can also be performed in people who are not intubated. A meta-analysis of Awake prone positioning in COVID has been shown to likewise improve oxygenation and reduce the need for intubation - 55 fewer intubations per 1000 patients - though awake proning probably doesn’t appear to reduce mortality.
4. Prednisone (But really we mean Dexamethasone)
Corticosteroids reduce inflammation and therefore are beneficial in a disease of lung inflammation like ARDS.
Specifically, corticosteroids reduces mortality (RR 0.84), ventilator days (4 days fewer), ICU LOS (1 day fewer), and hospital LOS (8 days fewer).
In order to be effective, it is important to use the correct corticosteroid at the correct dose at the correct time.
The current consenus is that dexamethasone at moderate dose given early is ideal.
Other corticosteroids have more mineralocorticoid effects which may promote fluid retention.
Higher doses may cause more side effects.
Late administration is not associated with benefit.
The Definitive study was DEXA-ARDS
Mutli-center RCT in spain that randomized 277 patients to either dexamethasone or placebo. Found substantially more ventilator-free days with dexamethasone (4.8 more day off the ventilator), as well as lower 60 day mortality (21% vs 36%). Both findings were significant despite the trial only achieving 80% of target enrollment.
Based in this crucial study, the recommended steroid in ARDS is: Dexamethasone 20 mg IV x 5 days, then 10 mg IV x 5 days.
There are different steroid recommendations depending on the etiology. If there is another specific indication (COVID, PJP, CAP) use that dose.
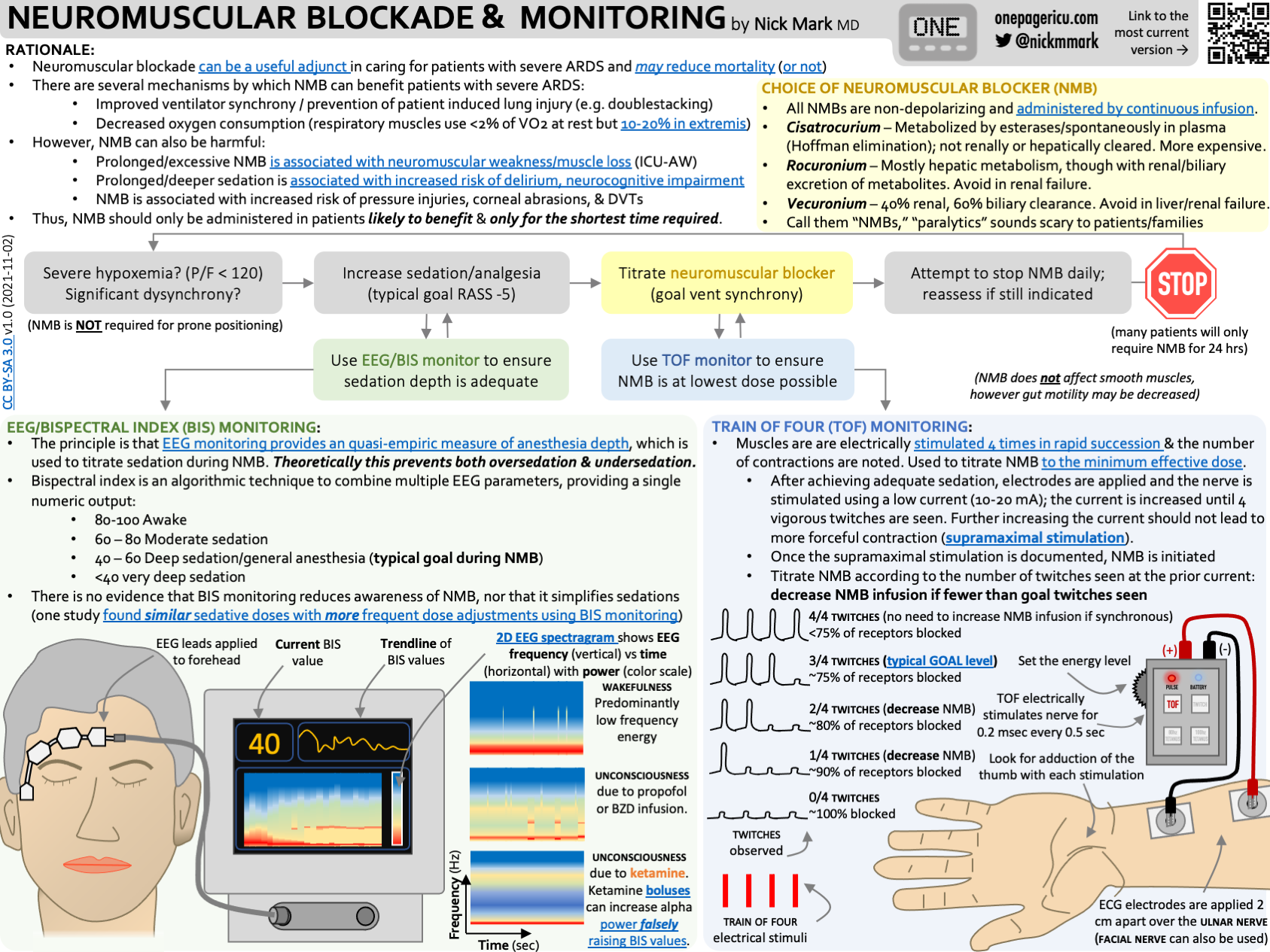
5. Paralytics (but please call them NMBs)
Neuromuscular Blockers - colloquially called paralytics - can improve ventilator synchrony & hypoxemia in severe ARDS.
There are two reasons why neuromuscular blockers (NMBs) are probably beneficial in ARDS: improved ventilator synchrony and reduced oxygen consumption.
Increase ventilator synchrony -
Remember that VILI can worsen lung injury in ARDS. If a patient is set to receive 6 ml tidal volumes but is double-stacking (meaning they are triggering another breath before exhalation completes) they could be getting as much as 12 ml/kg. Way too much!
NMBs remove patient triggered breaths and prevent this type of VILI.
Decrease oxygen consumption -
Using accessory muscles to breath consumes oxygen. In extremis, muscles of respiration can use up to 10-20% of our cardiac output, worsening hypoxemia.
NMBs take away this effort and therefore improve oxygenation.
Choice of NMB largely depends on how the drug is metabolized:
Rocuronium is hepatically cleared
Vecuronium is renally cleared.
Cisatrocurium is cleared by an organ independent process (hoffman degradation) spontaneously in the blood. In principle you could choose based on liver and kidney function. In practice, we usually default to cisatrocurium because so many sick ARDS patients have AKI and/or liver injury.
Initial studies suggested that paralytics might improve survival, but subsequent meta-analysis of multiple RCTs suggests probably not. Important studies include ACURASYS & ROSE.
There are significant downsides of using NMBs, which may explain the lack of robust survival benefit
First, deep sedation without daily awaking trials is required in order to give NMBs. We know that this comes with big downsides.
Second, if NMBs are working if means your muscles aren’t! This can contribute to significant muscle atrophy, especially if NMBs are used for prolonged periods.
If we are going to use NMBs to care for people with ARDS, we need to use them right. This means with appropriate monitoring & for the shortest time possible.
Train of Four (TOF) monitor - used to avoid too high a dose.
The device delivers four electrical stimuli to arm muscles.
Normally, this would cause four visible muscle contractions or twitches.
In someone who completely neuromuscularly blocked, this elicits zero twitches.
Ideally our goal is to achieve ventilator synchrony but still have 2/4 twitches, meaning that we haven’t “overdone it”
The concern with 0/4 twitches is that you don’t know if you are on just enough NMB drip or if you are on way too much.
BIS monitor - often used (without good evidence) to ensure that depth of sedation is sufficient.
Another concern is if someone is neuromuscularly blocked and inadequately sedated they could experiencing awareness under anesthesia.
The bispectral index is a device developed for use in the OR, to measure EEG activity and quantify it into a number from 0-100 that correlates with level of consciousness.
There is little evidence that BIS monitoring reduces awareness under anesthesia in the OR and no evidence for it’s use in the ICU.
Neuromuscular Blockers, Bispectral index (BIS), and Train-of-Four (TOF) Monitoring. By Nick Mark, MD and available at ICUOnePager
Myths about neuromuscular blockers:
“Hold tube feeds while on NMBs” - NMBs do NOT affect smooth muscle and thus the gut should be unaffected.
We can - and should - continue tube feeds while on NMBs.
“NMBs are required for proning or severe ARDS” - Just because someone has severe ARDS doesn’t mean they need to be on NMBs!
Even If someone is hypoxemic with significant dyssynchrony, remember that NMBs are the nuclear option. There’s a lot we can do to optimize the sedation, analgesia, and ventilator settings.
Some people only need NMBs transiently - one or two push doses instead of continuous infusion may be sufficient in many.
Not everyone who is prone requires NMBs, though they are often used together.
Although NMBs can be effective it’s really important to be aggressive about weaning off NMBs promptly.
Ideally - and according to some recent guidelines - you should only use NMBs for up to 48 hours.
6. “Prostacyclins” (inhaled prostacyclins or really inhaled pulmonary vasodilators)
Inhaled prostacyclins or inhaled nitric oxide have been shown to improve oxygenation in ARDS.
Rationale:
In ARDS many alveoli fill with fluid and cellular debris and that these “filled in alveoli” don’t participate in gas exchange.
Inhaled vasodilators go to ventilated alveoli (those that aren’t full of fluid) and vasodilate the blood vessels there. This increased perfusion to the better ventilated areas of the lung, improves V/Q matching.
Secondarily, many people with ARDS develop RV failure - in some studies as much as 10-25% - and pulmonary vasodilators help the the right ventricle by reducing pulmonary afterload or PVR, which makes it easier for a struggling RV to pump.
The data on outcomes suggests that inhaled prostacyclins improve oxygenation and reduce pulmonary pressures, but probably don’t reduce mortality.
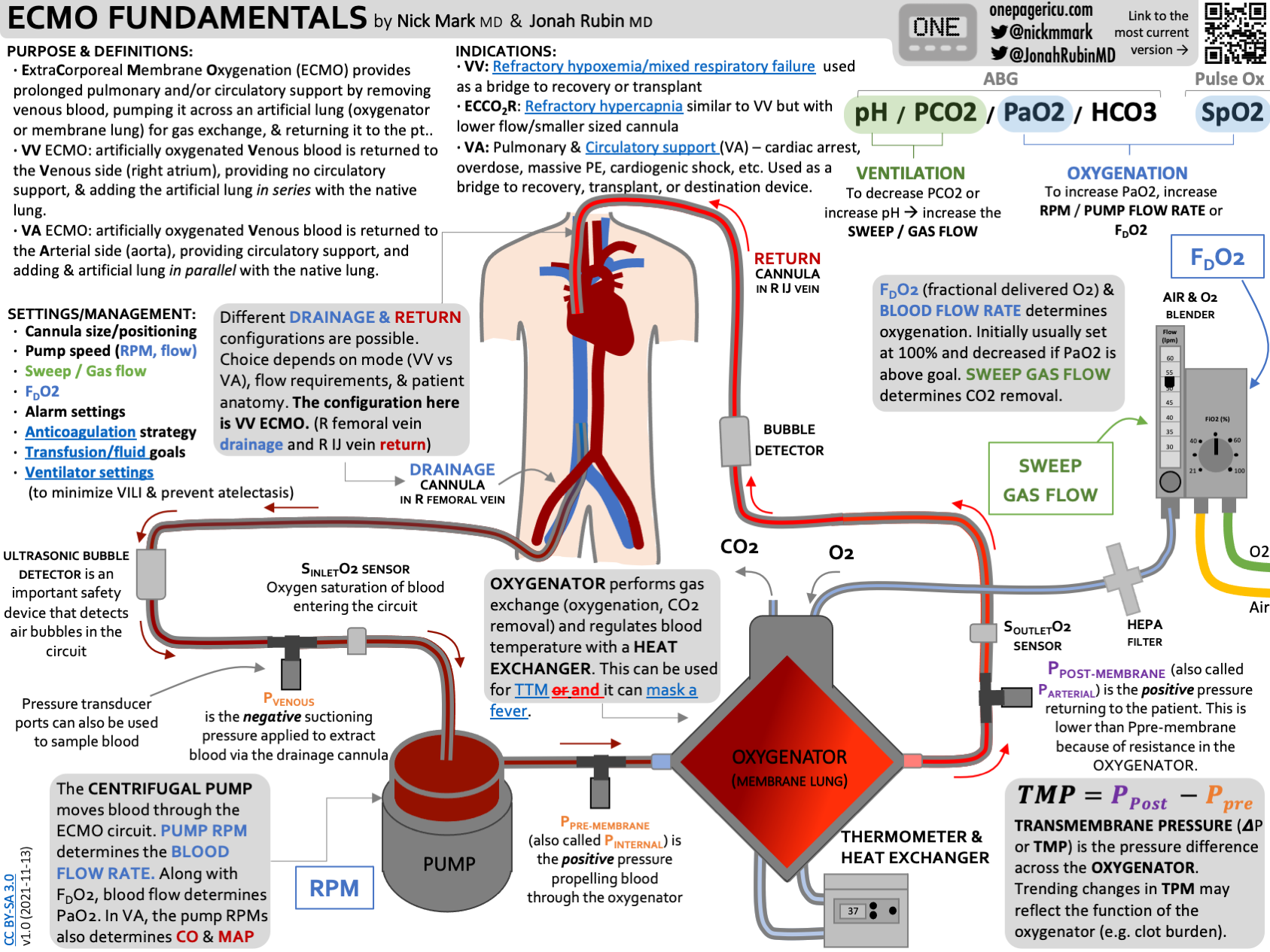
6. Peripheral Oxygenation (vvECMO)
In severe cases of ARDS, peripheral oxygenation - also known as vvECMO - can be lifesaving. However ECMO is a highly invasive procedure with significant procedural risks (cannulation, bleeding, limb ischemia, etc) so careful patient selection is essential.
Fundamentally, blood is removed from a drainage catheter, pumped through a membrane lung, where oxygen is added and CO2 removes, and then returned to the patient via a second cannula.
Two definitive trials in ECMO for ARDS were CESAR and EOLIA.
The Conventional ventilatory support vs Extracorporeal membrane oxygenation for Severe Adult Respiratory failure (CESAR) was a multi-center RCT in the UK that enrolled patients with severe ARDS whose Murray (composite score looking at compliance, PEEP, FiO2 and more) score was > 3 or a pH < 7.2 who were already on optimum conventional management.
Once patients were deemed sick enough to be eligible for ECMO based on those criteria, they were randomized to either the one ECMO hospital in the region or one of the other major referral centers, unless they were already at one of those referral centers in which case they would not be transferred
The primary outcome was Death or severe disability (defined as confinement to bed and inability to wash and dress alone) at 6 months.
Survival free of disability was significantly HIGHER in the cohort randomized to receive care in the ECMO center: 63% vs to 47% (which is a relative risk 0·69) for death or disability.
NNT to save a life is 7!
Importantly only about 75% of those randomized to ECMO actually received it.
EOLIA was a multi-center RCT at 64 hospitals in France, that randomized n=249 patients who were intubated for <7 days and despite optimal ventilation had either
A PaO2:FIO2 ratio <50 mmHg for >3 hours; or,
A PaO2:FIO2 <80 mmHg for >6 hours; or,
An Arterial blood pH <7.25 with PaCO2 >60 mmHg for >6 hours
EOLIA did allow cross-overs if people deteriorated in the control arm; this occurred in 28% of patients in the control arm, which was higher than anticipated when they powered the study.
For it’s primary outcome, EOLIA found 35% mortality with ECMO vs 46% with control, which did not quite reach statistical significance. (P=0.09)
Relative risk of mortality, 0.76; CI 0.55 to 1.04;
For secondary outcomes, EOLIA found less RRT in ECMO group there was also lower relative risk of treatment failure 0.62 (95% CI, 0.47 to 0.82; P<0.001) which was defined as death in the ECMO group or either crossover or death in patients in the control group.
Although technically “negative” EOLIA was highly suggestive of benefit to ECMO in ARDS.
Cross-over rate was much higher than expected.
EOLIA had a target enrollment of n=331 patients, but was stopped early after just n=249.
This likely would have been a “positive” trial if there had been less cross-over and if the study had been fully enrolled.
For more see the upcoming episode on ECMO Basics:
“Dry lungs are happy lungs”
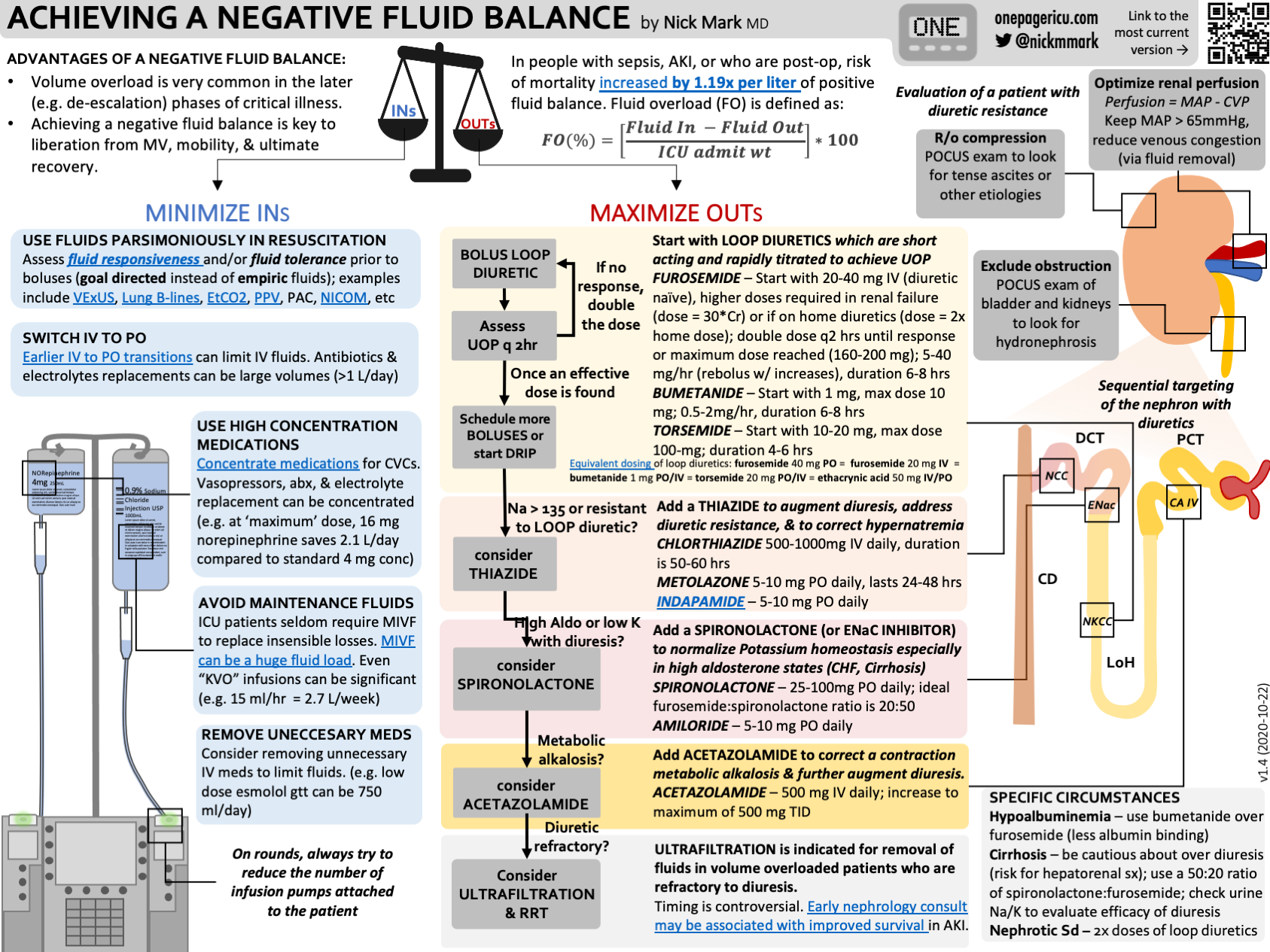
7. Parsimonious fluids & 8. Peeing (Diuresis)
Avoiding volume overload and aggressive diuresis have been shown to improve outcomes in ARDS.
Definitive Trial: FACTT trial was a 1000 person mutli-center RCT that randomized patients to conservative vs liberal fluid protocols.
In the liberal-strategy group, a CVP of 10 to 14 mm Hg and a PAOP of 14 to 18 mm Hg; in the conservative-strategy group, a CVP of less than 4 mm Hg and a PAOP of less than 8 mm Hg
They began using loop diuretics once the patients were hemodynamically stable which they defined at MAP > 60 and not requiring vasopressors.
At the end of 1 week, the liberal fluid group was positive almost 7000 ml whereas the conservative group was negative around 100 ml.
The mean daily dose of furosemide was about 150 mg in the conservative group.
The study found that a conservative fluid strategy - one that used more diuretic to acheive a lower CVP or Wedge pressure - was associated with several improvements in outcome. This included:
Shorter duration of mechanical ventilation:
Mean: 10 vs. 14 days (P<0.001)
Median: 6 vs. 9 days (P<0.001)
More ICU free days.
And a numerically lower rate of requiring dialysis. (10 vs 14%, p=0.06)
Although PAC were used to measure wedge pressure (PAOP) in FACTT, it is by no means necessary to place a PAC in ARDS.
We discuss the approach to diuresis and fluid balance in episode #25:
ICU OnePager approach to acheiving a negative fluid balance.
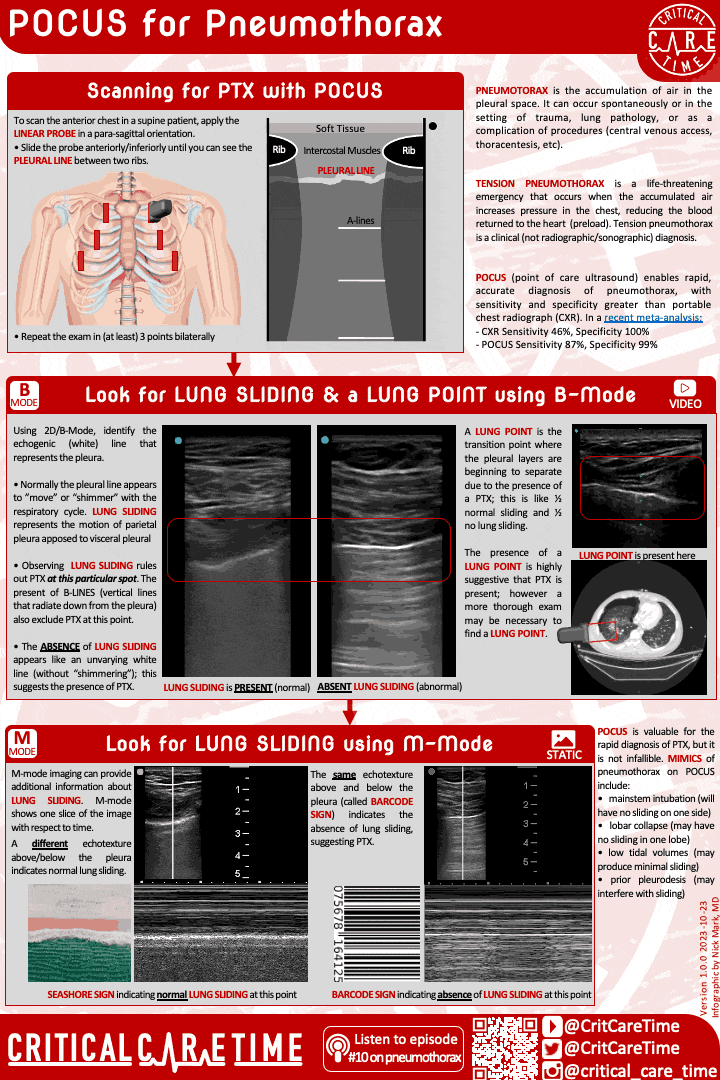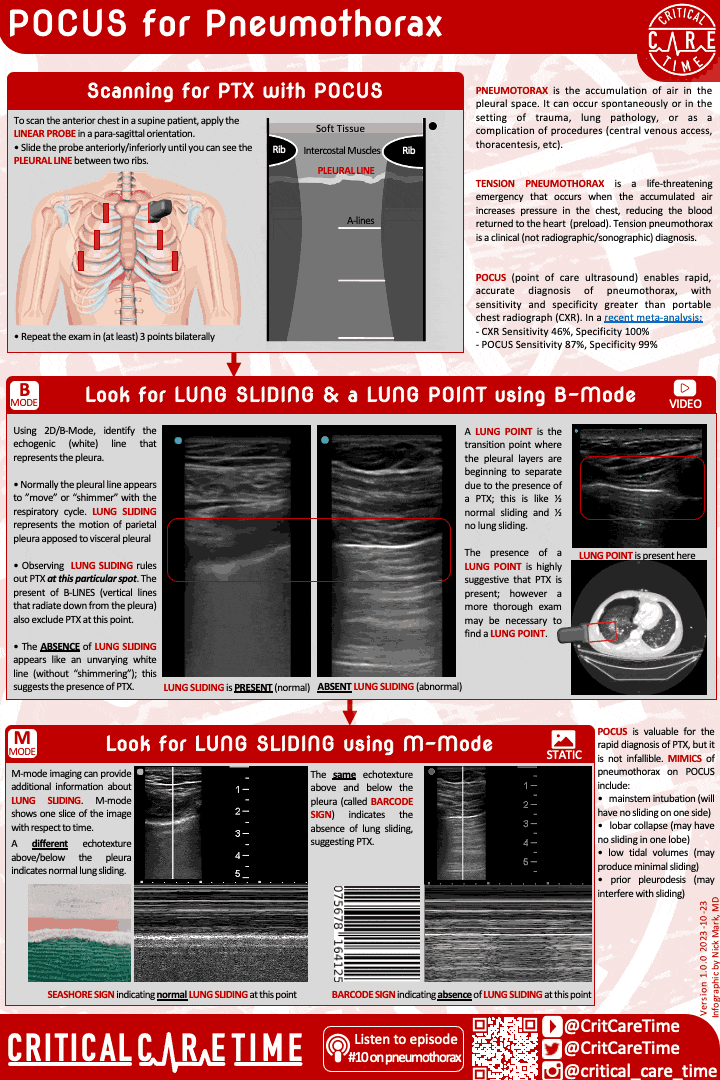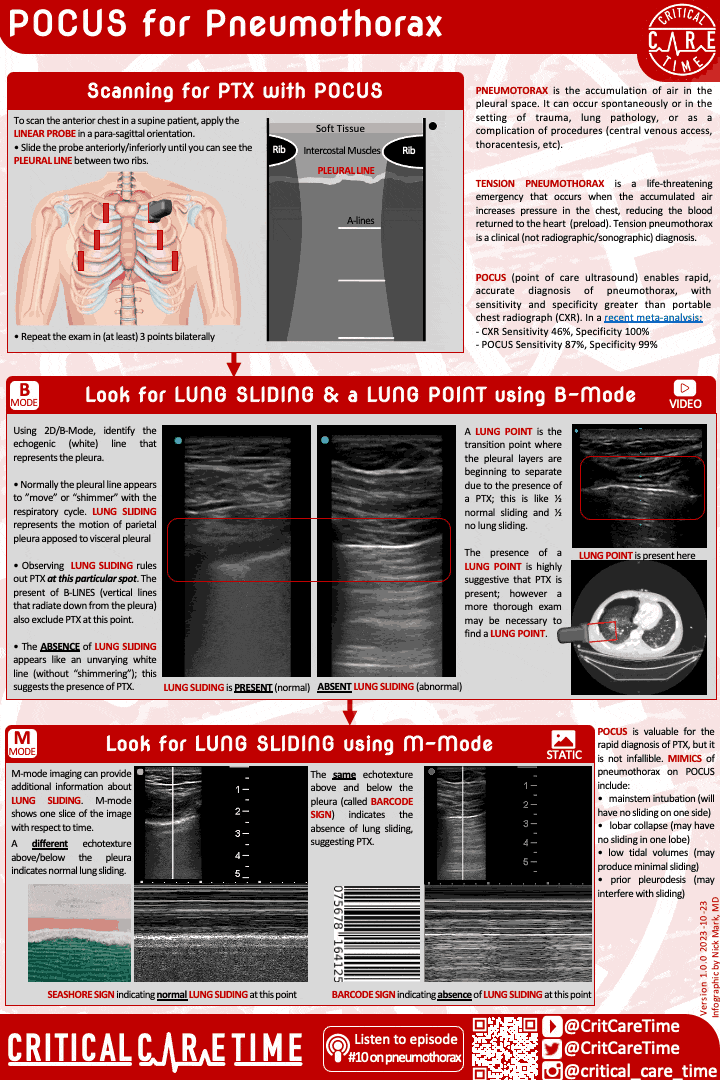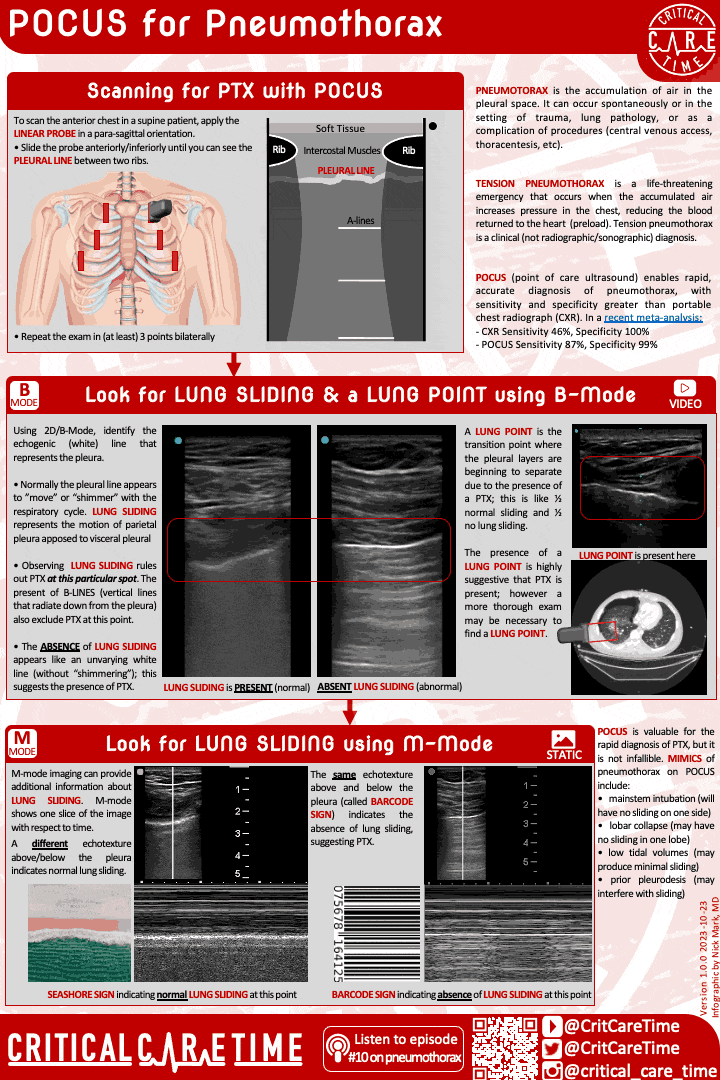
9. Pleural evacuation (recognition and treatment of PTX)
Pneumothorax is a common and highly fatal complication of ARDS. Pneumothorax can occur spontaneously and can rapidly become life threatening - especially in people on high PEEP and with higher driving pressures.
Have a high index of suspicion for PTX in ARDS. POCUS can be very useful in making this diagnosis quickly.
Contemporary studies - like of patients with ARDS due to COVID - suggest the rate of PTX in ARDS may be 8-10%.
A high index of suspicion is essential as early diagnosis is important. POCUS can be useful, though be cautious as lung sliding may be minimal in someone on very low tidal volume ventilation. When in doubt get a chest radiograph.
Small more chest tubes are ideal in PTX due to ARDS for evacuation of air.
For more see our earlier episode & infographic:
Critical Care Time infographic on using POCUS for recognizing pneumothorax.
10. Palliation
Early integration of palliative care can enhance decision-making about goals of care, reduce the intensity of interventions by aligning with patient wishes, and potentially improve overall quality of life for both patients and their families.
Although we’ve made significant progress in treating ARDS, mortality remains high & functional status after prolonged critical illness is often very limited. This may not be consistent with a person’s goals.
Palliation is not synonymous with withholding or withdrawing life support. Remember that palliation is about supporting patients and families, which is really important when dealing with a often prolonged critical illness like ARDS.
We’ll talk much more about the importance of palliative care in an upcoming episode.
-
Ashbaugh DG et al, Acute Respiratory Distress Syndrome in Adults, The Lancet 1967
Papazian L et al, Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome, NEJM 2010 (ACURASYS)
Brower RG et al, Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. NEJM 2000 (ARMA Study)
Villar J et al, Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020 (DEXA-ARDS)
Amato MB et al, Driving pressure and survival in the acute respiratory distress syndrome. NEJM 2015
Wiedemann HP et al, Comparison of two fluid-management strategies in acute lung injury.NEJM 2006 (FACTT)
Combes A et al, Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome.NEJM 2018 (EOLIA)
Peek, Giles J et al, Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial, The Lancet 2009 (CESAR)
Guérin C et al, Prone Positioning in Severe Acute Respiratory Distress Syndrome, NEJM 2013 (PROSEVA)
Moss M et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. NEJM 2019 (ROSE)
-
Hypoxemia